Research
Several research directions in our lab:
1) One of our main research interests is the direct coupling of organic molecules through transition-metal-catalyzed C-H activation. We are especially interested in developing catalytic systems that recognize/activate the substrate and enable site-selective functionalization.
2) We pursue the development of sustainable organic synthesis using Earth-abundant metals as catalysts.
3) We believe in the renaissance of organosodium chemistry, and we explore the synthesis and synthetic applications of organosdium compounds, as a sustainable alternative to organolithium.
Recent Research Results
Ligand-enabled site selective C–H functionalization
Remote steric control with roof SpiroBipyridine ligands
Science 2022, 375, 658–663. pdf press release research highlight
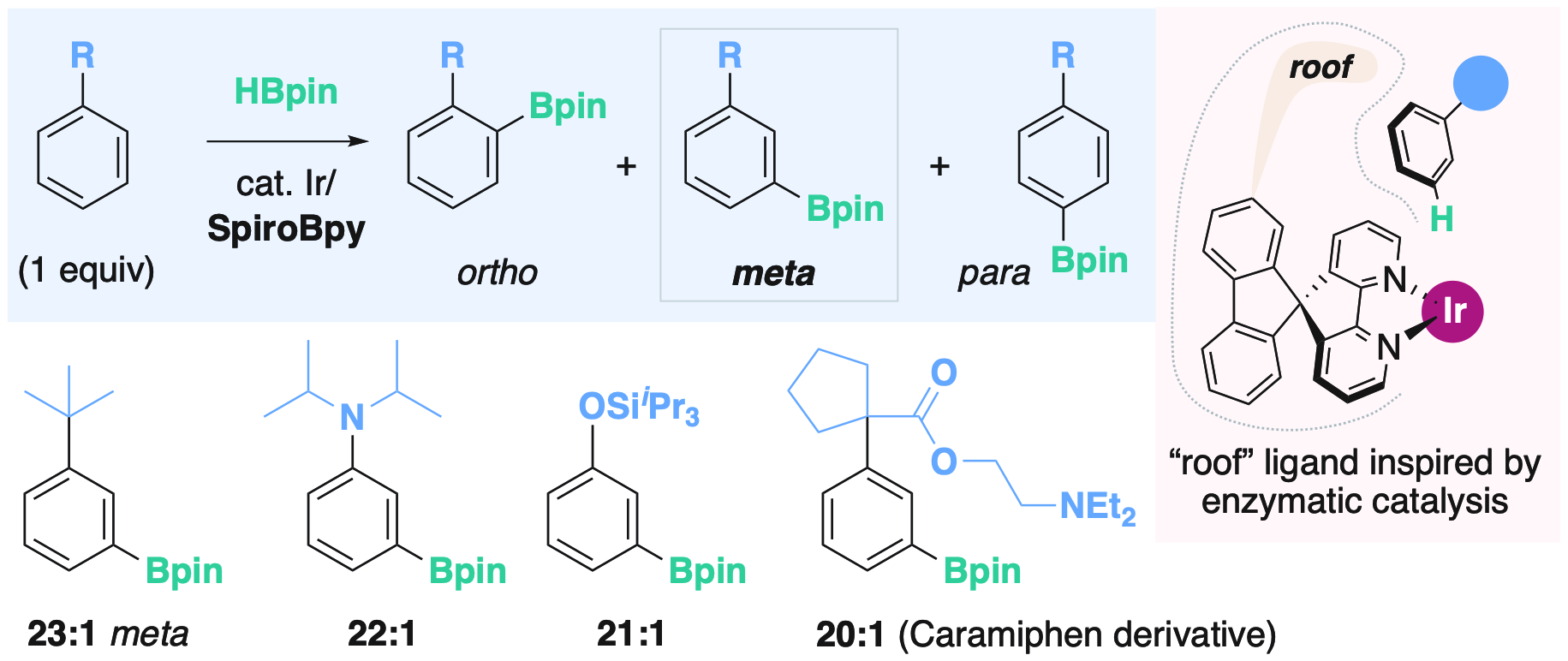
A major goal of modern synthetic chemistry is the creation of organic molecules in the most straightforward and efficient manner possible. For this purpose, the use of a metal catalyst to directly introduce functionality into a simple or complex organic molecule has received much attention. However, a serious problem remains largely unsolved: even simple organic molecules have many reaction sites, and selective reaction at the desired site is very difficult. For example, any student of introductory organic chemistry has encountered "trick" problems about ortho/meta/para selectivity in the reaction of arenes. Our research provides a new solution to this problem. Inspired by the lock-and-key model of enzymatic catalysis, we designed a new catalytic system that creates a molecular pocket to fit the substrate only in a determined orientation, and thus control the selectivity. We demonstrated this concept for the meta-selective functionalization of simple arenes such as alkylbenzenes, anilines, and phenols, and for the late-stage selective functionalization of complex drug molecules. The new method has the potential to efficiently synthesize molecules of interest for medicinal chemistry, materials science, etc., and to create new, unexplored chemical space for drug discovery or the development of functional materials
Remote steric control with roof SpiroBipyridine ligands
Science 2022, 375, 658–663. pdf press release research highlight
A major goal of modern synthetic chemistry is the creation of organic molecules in the most straightforward and efficient manner possible. For this purpose, the use of a metal catalyst to directly introduce functionality into a simple or complex organic molecule has received much attention. However, a serious problem remains largely unsolved: even simple organic molecules have many reaction sites, and selective reaction at the desired site is very difficult. For example, any student of introductory organic chemistry has encountered "trick" problems about ortho/meta/para selectivity in the reaction of arenes. Our research provides a new solution to this problem. Inspired by the lock-and-key model of enzymatic catalysis, we designed a new catalytic system that creates a molecular pocket to fit the substrate only in a determined orientation, and thus control the selectivity. We demonstrated this concept for the meta-selective functionalization of simple arenes such as alkylbenzenes, anilines, and phenols, and for the late-stage selective functionalization of complex drug molecules. The new method has the potential to efficiently synthesize molecules of interest for medicinal chemistry, materials science, etc., and to create new, unexplored chemical space for drug discovery or the development of functional materials
Selective borylation of fluoroarenes using terpyridine ligands
ACS Catal. 2021, 11, 5968–5973. link
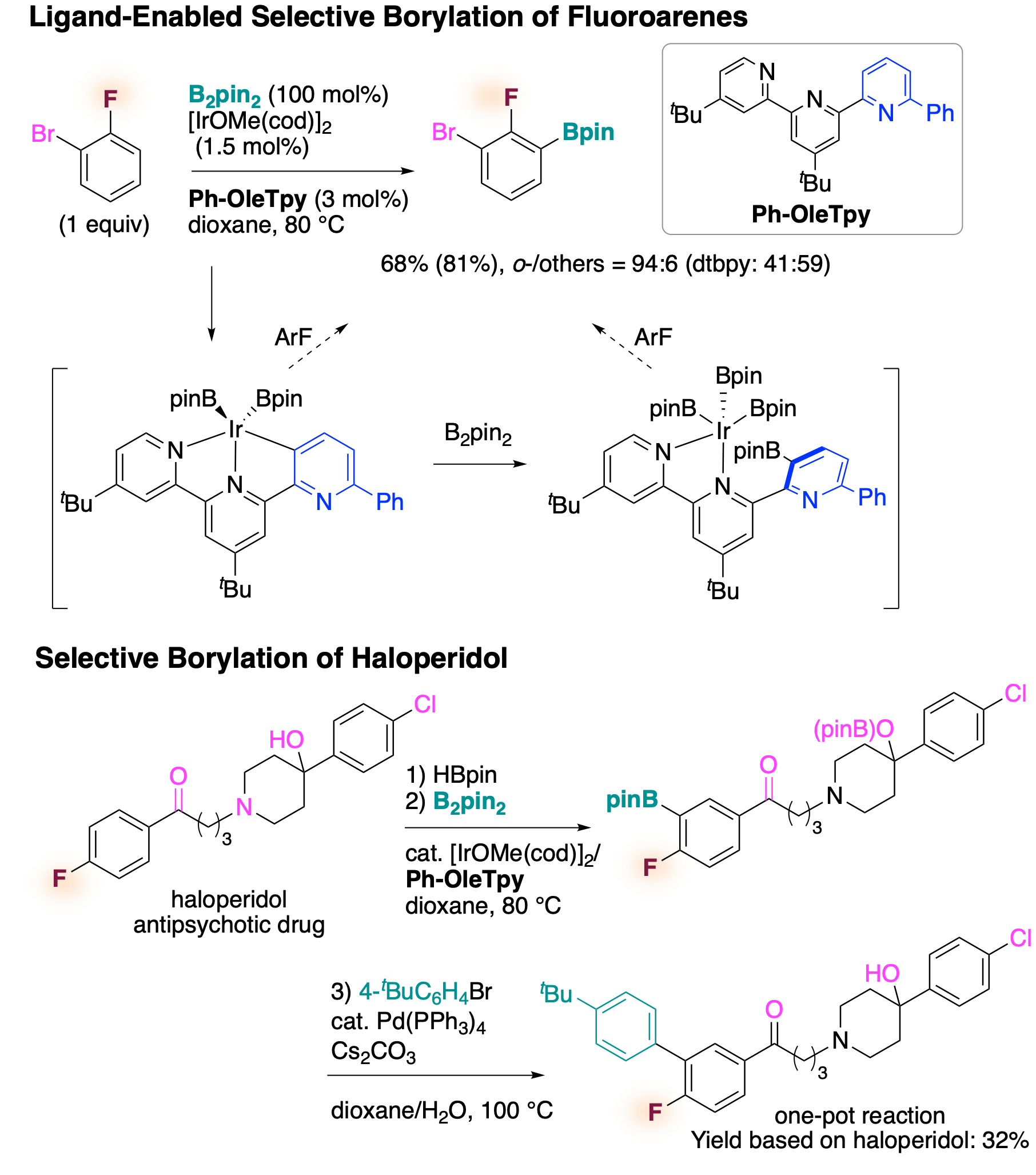
Fluoroarenes are important compounds for drug discovery, and late-stage selective functionalization of these compounds allows structure-bioactivity relationship studies. We developed a new terpyridine ligand, which in combination with an iridium salt catalyzes the stoichiometric borylation of various fluoroarenes with high ortho-selectivity to fluorine. The catalytic system tolerates sensitive functional groups, and thus could be used for the selective functionalization of a complex drug molecule such as haloperidol. Preliminary mechanistic studies by experiment and calculation suggested that the terpyridine ligand does not coordinate iridium through the typical N, N, N-coordination mode, but instead undergoes rollover cyclometalation to generate an N, N, C-coordinated iridium complex.
ACS Catal. 2021, 11, 5968–5973. link
Fluoroarenes are important compounds for drug discovery, and late-stage selective functionalization of these compounds allows structure-bioactivity relationship studies. We developed a new terpyridine ligand, which in combination with an iridium salt catalyzes the stoichiometric borylation of various fluoroarenes with high ortho-selectivity to fluorine. The catalytic system tolerates sensitive functional groups, and thus could be used for the selective functionalization of a complex drug molecule such as haloperidol. Preliminary mechanistic studies by experiment and calculation suggested that the terpyridine ligand does not coordinate iridium through the typical N, N, N-coordination mode, but instead undergoes rollover cyclometalation to generate an N, N, C-coordinated iridium complex.
Synthesis of organosodium compounds through halogen/sodium exchange
Commun. Chem. 2021, 4, 76. link
Review: Synthesis 2021, DOI: 10.1055/a-1478-7061. link
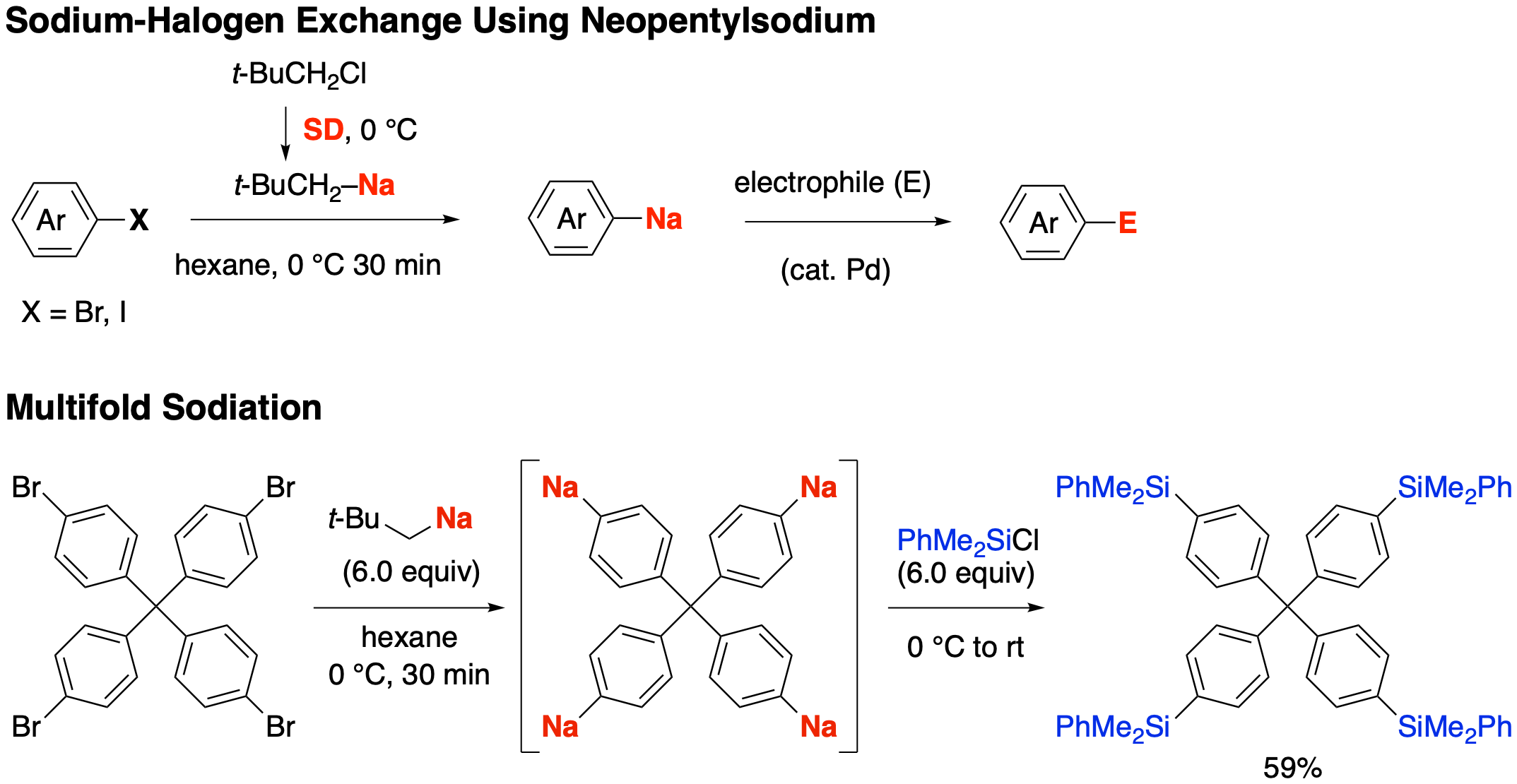
Halogen–lithium exchange has been extensively used in organic synthesis because it allows rapid preparation of a large variety of organolithium compounds from (hetero)aryl or alkenyl bromides or iodides, typically using butyllithium or tert-butyllithium under cryogenic conditions. From a sustainability point of view, there is a growing demand for alternatives to the less abundant and increasingly expensive lithium. Sodium is an attractive candidate, but attempts at halogen–sodium exchange using alkylsodiums such as butylsodium and pentylsodium have met with significant challenges, such as low yields and formation of byproducts.
We discovered that the use of a primary and bulky alkylsodium lacking β-hydrogens retards undesired reactions and enables efficient halogen–sodium exchange. The alkylsodium is readily prepared in situ from neopentyl chloride and an easy-to-handle sodium dispersion, and allows us to prepare a large variety of (hetero)aryl- and alkenylsodium compounds including tri- and tetrasodioarenes, many of them previously inaccessible by other methods.
We believe that this reaction has the potential to replace the textbook halogen–lithium exchange, and open new frontiers for establishing organosodium-based organic chemistry.
Commun. Chem. 2021, 4, 76. link
Review: Synthesis 2021, DOI: 10.1055/a-1478-7061. link
Halogen–lithium exchange has been extensively used in organic synthesis because it allows rapid preparation of a large variety of organolithium compounds from (hetero)aryl or alkenyl bromides or iodides, typically using butyllithium or tert-butyllithium under cryogenic conditions. From a sustainability point of view, there is a growing demand for alternatives to the less abundant and increasingly expensive lithium. Sodium is an attractive candidate, but attempts at halogen–sodium exchange using alkylsodiums such as butylsodium and pentylsodium have met with significant challenges, such as low yields and formation of byproducts.
We discovered that the use of a primary and bulky alkylsodium lacking β-hydrogens retards undesired reactions and enables efficient halogen–sodium exchange. The alkylsodium is readily prepared in situ from neopentyl chloride and an easy-to-handle sodium dispersion, and allows us to prepare a large variety of (hetero)aryl- and alkenylsodium compounds including tri- and tetrasodioarenes, many of them previously inaccessible by other methods.
We believe that this reaction has the potential to replace the textbook halogen–lithium exchange, and open new frontiers for establishing organosodium-based organic chemistry.